GOTHAMIST
Editor's note: The Centers for Disease Control and Prevention recommended the Pfizer vaccine for children ages 5 to 11 on November 3rd, launching the rollout.
Children ages 5 to 11 will likely be able to get their COVID-19 shots next month after an advisory committee to the Food and Drug Administration voted in favor of authorizing the Pfizer vaccine for all children in this age group on Tuesday. Moderna also released preliminary data this week on how its vaccine performs among younger kids and announced its intentions to seek a similar authorization.
For some New York parents, the shots can’t come soon enough
.
“We've been keeping an eye out, hoping that it would be approved for their age group,” said Robert Currie, a resident of Fort Greene, Brooklyn, whose children are 6 and 9 years old. Currie is from Scotland and said being unable to get his kids vaccinated made him worry more about traveling internationally with his family. Those 12 and older have been eligible since May.
“It’s added another complicating factor to something that was already complicated,” he said.
Others are more hesitant — in some cases, even if they have been vaccinated themselves.
“It's a hard decision,” said Liscole Young, whose 6-year-old twins attend Elijah G. Stroud Elementary in Crown Heights. She said her whole family, including her 15-year-old, have been vaccinated against the coronavirus, except the twins. She wants to protect them but has become skeptical about the effectiveness of the vaccines because of reports of breakthrough cases — where people catch the virus despite being inoculated.
“I do feel as though they will get it,” she said. “But if they debut it tomorrow, I won't be the first in line. I might not even be the 10th person in line.”
Even eager parents might still have questions about the findings from Pfizer’s clinical trial, where the shots will be available in New York, and whether taking the doses will be required for going to school. The FDA committee itself had long deliberations about whether the shots should be for all kids or just ones with pre-existing conditions. WNYC/Gothamist reached out to vaccine experts, pediatricians and city and state health officials to get their input.
I thought children were less likely to get COVID. Does my kid really need a shot?
It’s true that confirmed COVID-19 cases were less common among children earlier in the pandemic, potentially because schools and daily life were locked down. But cases are on the rise for the youngest Americans. More than 1 million children nationwide have received a positive test result since the first week of September, according to the American Academy of Pediatrics. And kids made up more than a quarter of coronavirus cases diagnosed between October 15th and October 21st, about on par with their proportion of the population.
That said, kids are still less likely than adults to be symptomatic, require hospitalization or die from COVID-19
“Think of it from the perspective of the virus: Who’s available for infecting?” said Dr. Jessica Justman, associate professor of medicine at Columbia Mailman School of Public Health. “A lot of the older people who’ve been vaccinated are much less available. It’s really no surprise that the case rate should begin to tilt toward those who are younger.”
While serious illness is uncommon, more than 5,000 children have suffered a rare complication known as multisystem inflammatory syndrome (MIS-C). And some children are also developing the chronic syndrome known as Long COVID, with a wide range of symptoms that persist well after infection.
Right now in New York City, school-age children have the highest COVID case rates of any age group. More than 4,400 students in NYC schools have tested positive since the start of the 2021-2022 school year. And 30 children in NYC have died of COVID over the course of the pandemic.
“Getting COVID for kids is a much worse prospect than getting the vaccine,” said Dr. Denis Nash, professor of epidemiology at the City University of New York. “The virus causes organ damage in kids, hundreds of thousands of kids have been hospitalized, more than 100,000 have been orphaned. The vaccine is really the way forward, out of this very unfortunate scenario.”
What is the authorization process like?
It started with Tuesday’s meeting of the FDA’s Vaccines and Related Biological Products Advisory Committee. Its members — a collection of nationwide scientists — reviewed the Pfizer data, discussed the possibility of heart inflammation in childhood recipients given past observations in young adults and weighed the risks and benefits of vaccinating kids in this age group. Now that the panel has voted on a recommendation, the acting FDA commissioner Dr. Janet Woodcock and her staff will decide whether to issue an emergency use authorization.
Next week, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will hold a meeting to propose guidelines for who should get the vaccine. CDC director Rachelle Walensky has the final say on the scope of the authorization.
What do we know about the Pfizer vaccine for children?
According to data shared with the FDA, Pfizer’s clinical trial included 2,268 5- to 11-year olds, 1,528 of whom received two kid-sized doses of the Pfizer-BioNTech vaccine — about one-third the amount of an adult dose. The rest got two shots of a placebo. The researchers kept up with the kids for two months after the second dose — tracking their antibody levels, COVID cases and adverse reactions. The study didn’t record any hospitalizations in the placebo group or those who took the shots, so it’s unclear how the vaccine performs against severe COVID-19 in kids.
The trial found that the reduced dose triggered an immune response comparable to that of the full dose in teens and young adults. All told, 16 children in the placebo group tested positive for COVID-19 during the follow-up period, compared with just three of the kids who received both doses of the vaccine. That means the vaccine was about 91% effective against catching a symptomatic case of the coronavirus.
How about side effects?
Reactions after the shots are on par with what we’ve seen in older kids and adults, or sometimes even milder. Soreness at the injection site was common, happening about 70% of the time — or almost the same rate witnessed among teenagers and young adults. Fewer kids reported fatigue (39%) and headaches (28%) — or about half the rates observed with teens and young adults. As usual, most of the side effects showed up in the first day or two after receiving a vaccine dose and abated quickly.
The researchers didn’t find any serious side effects during the study period, including myocarditis or pericarditis, two types of heart inflammation observed in a small fraction of teens and young adults who’ve received one of the two mRNA vaccines. Because myocarditis and pericarditis are so rare, a study of this size was unlikely to capture any instances of these reactions, Dr. Justman noted.
“It’s two months worth of observation; it’s not six months or two years,” she said. “But on the other hand, the vast majority of the side effects that have occurred in association with receiving the vaccine have been certainly within the two month period.”
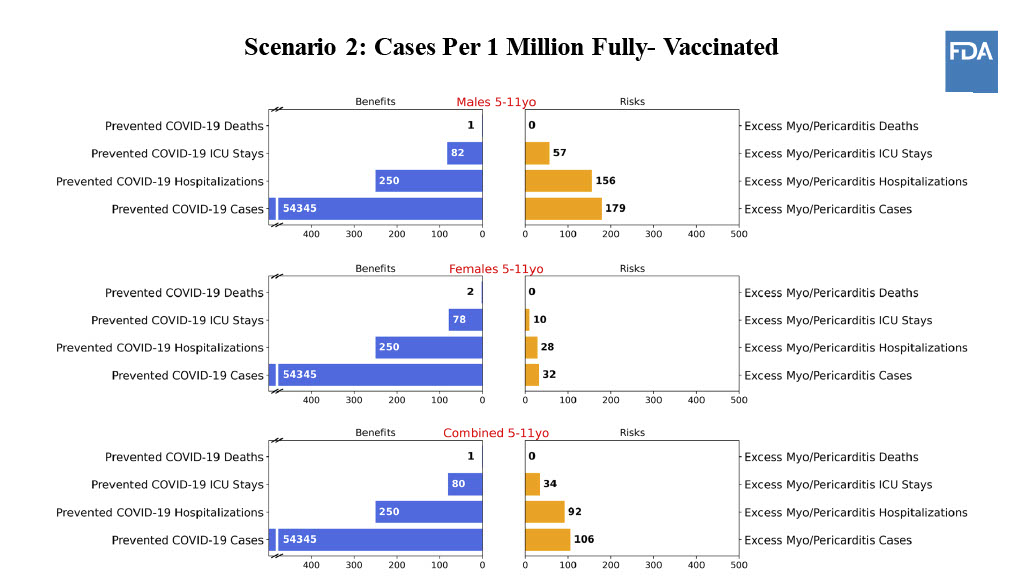
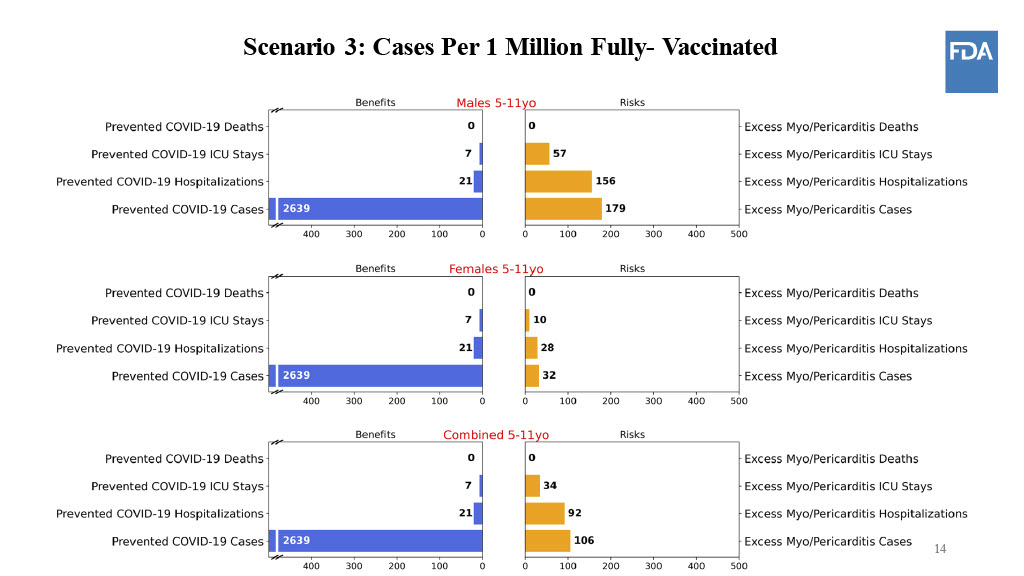
At Tuesday’s advisory meeting, researchers presented some hypothetical models to gauge the best-case and worst-case scenarios when it came to these heart conditions among kids under 12. The vaccines haven't been given to enough young kids to know their true rates of myocarditis, so in this model, the FDA assumed that children under 12 would experience myocarditis at similar rates as teenagers and young adults.
Put simply, if coronavirus cases among children rise substantially in the future and the vaccine lowers the chances of childhood hospitalizations at the same rate as adults, then the benefits outweigh the risks when it comes to taking the pediatric shots.
But if cases continue to decline, the benefits work more in favor of girls than boys — assuming that the same gender-based patterns in young adults also apply to children.
The advisory committee also floated the idea of authorizing the pediatric vaccine only for kids with pre-existing health conditions.
When and where will younger children be able to get the shot?
New York City and state officials say they are working to ensure young children can get vaccinated as soon as they become eligible.
“This includes significant planning and preparation with NYC vaccination sites and pediatric care providers who already know these children, as well as exploring other opportunities for youth-specific vaccination,” a spokesperson for the city health department said.
While many adults and teens have gotten vaccinated at city- and state-run sites, doctors’ offices will likely play a much bigger role in vaccinating young children.
“Everybody recognizes there's a major difference between vaccinating a five-year-old versus a 15-year-old,” said Dr. Warren Seigel, chair of the New York State chapter of the American Academy of Pediatrics. “Giving a 15-year-old a shot, they're less likely to be screaming. They're less likely to need to be held down. You really need that TLC [tender loving care] with a 5-year-old.”
Some doctors are already stocking up. Dr. David Buccholz, a pediatrician in Manhattan, said his practice has put in an order to the city for the special vials of the vaccine that will be used for patients under 12.
Still, storing this vaccine at ultra-cold temperatures and making sure doses are administered in a timely fashion so they don’t go to waste can be a challenge for medical practices. Gov. Kathy Hochul said last week she’s working to ensure pediatricians have all the supplies, staff and support they need. “I want to make sure that the doctors' offices where I think the majority of parents will get those vaccines are ready for this," she said.
Will kids have to get inoculated against the coronavirus to go to school?
Mayor Bill de Blasio has made it clear that he will not make getting vaccinated against COVID-19 a condition for attending public school, even though kids are required to get several other vaccines.
“
I believe at this moment, especially, the key is to get kids in school,” de Blasio said last week. “We still see too much misinformation out there about the vaccine, which means parents who might not let their kid get vaccinated, even if it's in the kid's interest, and the kid couldn't go to school.”
But de Blasio’s term is up at the end of the year and Eric Adams, the favorite in the mayoral election, has said he would require kids to get coronavirus shots to go to school, pending their approval by the FDA. Pfizer shots currently have full approval for people older than 16. Otherwise, the vaccines currently available in the U.S. have emergency use authorization. Curtis Sliwa, who is running against Adams, has come out against vaccine mandates for all ages.
Meanwhile, the New York chapter of the American Academy of Pediatrics is urging the state to require all children attending school, daycare and after-school activities to get vaccinated against the coronavirus once the shots are rubber stamped. Hochul responded at a press conference last week that she is “certainly willing to look at this,” noting that California will require kids to have their coronavirus shots for the next school year.
Are parents ready to get young children vaccinated against COVID-19?
Some pediatricians and epidemiologists are concerned that parents will be less willing to vaccinate their youngest children, while others say they anticipate questions and concerns similar to those that came up when the shots first became available for adults and adolescents. So far, about two-thirds of 13- to 17-year-olds are vaccinated citywide.
A survey conducted in New York City in March found that about 62% of 1,119 caregivers would vaccinate their children under 12 when they became eligible, whereas a national survey conducted the same month found that only about half of respondents would get their kids immunized.
A more recent national poll by Ipsos released this month found that 67% of parents were “very” or “somewhat” likely to vaccinate their children under 12. Surveys have found that parents who are vaccinated themselves are far more likely to be willing to vaccinate their kids. Those who are hesitant are generally concerned about the safety and efficacy of the shots.
Doctors say they are already fielding questions.
“This is no different than what pediatricians have been doing before the pandemic,” said Dr. Seigel. “Remember, there's a recommendation for children five and up to get an annual flu shot. We deal with vaccine questions and vaccine hesitancy every day.”